Key Differences Between (Corrosion and Thermal) Fatigue Cracking
- matintegrity
- Jun 24, 2023
- 3 min read
In the realm of materials science and engineering, two significant phenomena that can compromise the structural integrity of components are corrosion fatigue cracking and thermal fatigue cracking. While they share some similarities, understanding their key differences is crucial for effective analysis, prevention, and mitigation strategies. Let's explore these two forms of damage and their distinguishing characteristics.
Corrosion fatigue cracking occurs when a material is subjected to cyclic loading in a corrosive environment. It is a synergistic process where the combined effects of cyclic stresses and corrosion lead to crack initiation and propagation. The presence of a corrosive medium, such as moisture or aggressive chemicals, weakens the material's protective oxide layer, making it more susceptible to corrosion, initiating localized corrosion sites which then act as initiation point for cracks. As cyclic loads are applied, cracks develop at these corrosion sites, which can eventually propagate and lead to catastrophic failure.
On the other hand, thermal fatigue cracking arises from the repetitive heating and cooling cycles that a material experiences. It occurs when components are exposed to significant temperature variations, leading to differential expansion and contraction. This thermal cycling induces localized stresses, causing cracks to initiate and propagate over time.
Unlike corrosion fatigue cracking, thermal fatigue cracking does not require the presence of a corrosive environment.
Thermal fatigue is the result of cyclic stresses caused by variations in temperature. Damage is in the form of cracking that may occur anywhere in a metallic component where relative movement or differential expansion is constrained during repeated thermal cycling.
The cracks formed due to thermal fatigue typically exhibit a transgranular or intergranular pattern, following the crystallographic structure of the material.
One of the key distinctions between these two types of cracking is their primary driving factors. Corrosion fatigue cracking is influenced by the combined effects of mechanical stress and corrosion, while thermal fatigue cracking is predominantly caused by temperature fluctuations and the resulting thermal stresses. Understanding the specific mechanisms behind each type of cracking is essential for selecting appropriate prevention and mitigation strategies.
Another important difference lies in the environmental conditions that contribute to the occurrence of these phenomena. Corrosion fatigue cracking requires the presence of a corrosive medium, such as moisture or chemicals, which accelerates the corrosion process and enhances crack initiation. In contrast, thermal fatigue cracking can occur in various environments, as it is primarily driven by temperature fluctuations rather than corrosive agents.
The appearance and characteristics of the cracks formed during corrosion fatigue and thermal fatigue also differ. Corrosion fatigue cracks often exhibit a more branched and irregular pattern, with rougher surfaces due to the corrosive attack. In contrast, thermal fatigue cracks tend to have a more straight and uniform appearance, following the crystallographic planes of the material.
Lastly, the prevention and mitigation strategies for these types of cracking also vary. To mitigate corrosion fatigue cracking, it is crucial to address both the mechanical loading conditions and the corrosive environment. Strategies may include improving material selection, applying protective coatings, controlling environmental factors, and implementing proper maintenance practices. Thermal fatigue cracking prevention involves reducing temperature differentials, optimizing thermal insulation, and employing design techniques that minimize direct thermal exposure and promote uniform temperature distribution.
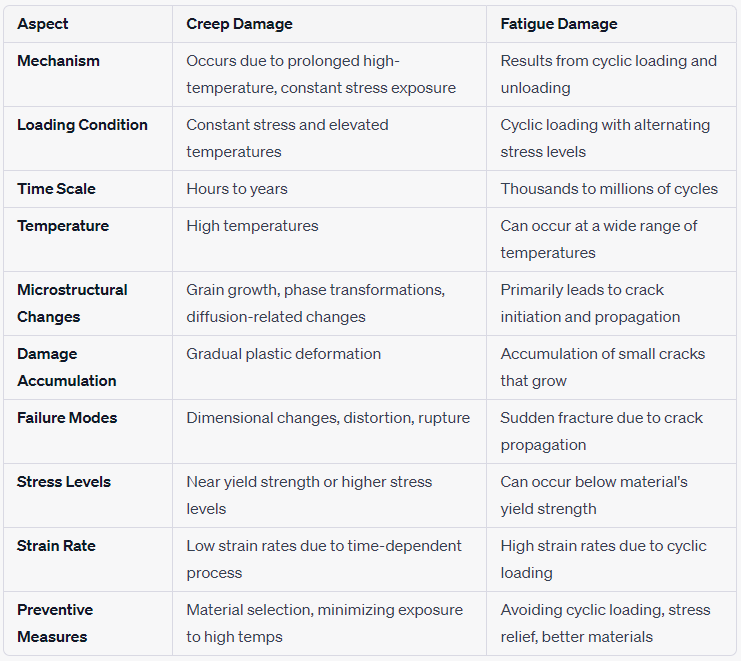
The below table provides a concise overview of the key differences between corrosion fatigue cracking and thermal fatigue cracking. However, it's important to recognize that these phenomena can have more complex aspects and considerations depending on specific materials, conditions, and applications.







Comments